Aluminum Battery Recharges in 1sec
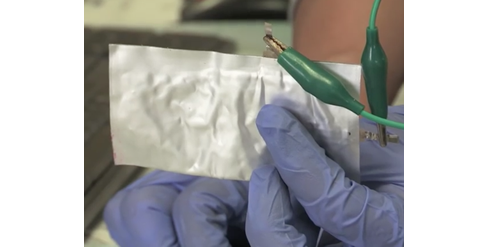
PORTLAND, Ore. — A flexible battery made from cheap, plentiful materials and which can recharge instantly is the Holy Grail of energy storage technology. Now Stanford University claims to achieved this goal. Called simply the “aluminum battery” it uses a flexible aluminum foil anode, a graphite foam cathode, liquid salt as an electrolyte and can be recharged in one second.
“People have worked on aluminum ion batteries, but they all had low voltages and poor cycle lifetimes. The aluminum/graphite battery was actually discovered by accident, but we were able to improve the performance once we knew that graphite was a good cathode candidate,” Ming Gong, a doctoral candidate working in professor Hongjie Dai’s labratory at Stanford University, told EE Times.
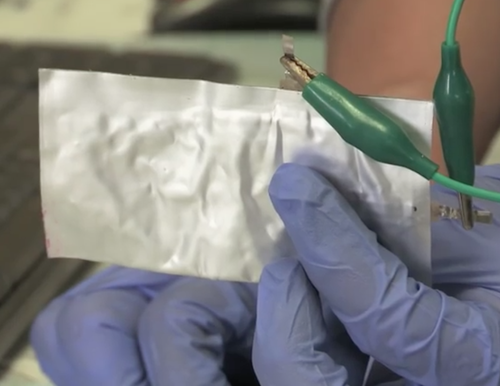
A porous graphite cathode appears to have been the secret sauce enabling the aluminum battery to rival lithium ion batteries (when they were first built in prototype). Today the aluminum battery produces only 2.5 volts, forcing two to be used in series to achieve the five volts that most devices require. And the capacity is one third of a lithium ion battery. But the Coulombic efficiency is high (>95%) giving the team optimism that its capacity and other desirable qualities can be improved just as the capacity of lithium-ion batteries has been improved by optimization.
(Source: Stanford)
Once of the quirky aspects of the flexible aluminum battery is that it uses liquid salt as its electrolyte, making it both cheap and nonflammable.
“The electrolyte is made of an ionic liquid (imidazolium-based). Mixing the two solid precursors — we call them EMIC (1-ethyl-3-methyl-imidazolium chloride) and AlCl3 (aluminum chloride) — makes them a liquid at room temperature as the melting point is significantly lowered,” Gong told us.
In addition, the porous graphite cathode both increases the amount of current the battery can deliver, as well as accounts for its super-fast recharging time.
“The graphitic foam is porous, so it has large surface area for accessing the electrolyte and consequently it has a low energy barrier to the intercalation process during charging,” Gong told us.
(Source: Stanford)
The biggest difference with lithium-ion batteries, beside being flexible and nonflammable, is that even the prototype can be recharged 7.5X more times than lithium-ion. Lithium-ion batteries typically can withstand only about 1000 recharges before becoming loosing significant capacity, whereas the aluminum battery can withstand at least 7500 recharges with no loss of capacity. They are also suitable for large installations, such as storing solar energy during the day for use at night on the grid.
Source: eetimes.com





